當前位置:首頁>商城動態(tài)
發(fā)布時間:2024/9/24 9:44:39 閱讀次數(shù):261
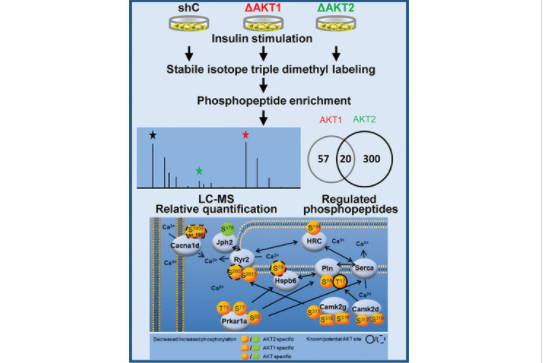 phosphoproteome of HL-1 cardiomyocytes carrying AKT1 or AKT2 isoform-specific knock down, respectively. We combined stable isotope labeling with high resolution mass spectrometry and identified 377 regulated phosphopeptides. Although AKT1 is expressed at 4-fold higher levels, insulin stimulation mainly activated AKT2, which might in part rely on a preferred interaction of AKT2 with the mammalian target of rapamycin complex 2. In line with this result, the highest number of regulated phosphopeptides was identified in the AKT2 knock down cells. Isoform-specific regulation of AKT targets not previously described could be observed, and specific regulation of indirect target sites allows a deeper insight into affected biological processes. In the myocardial context, we identified many phosphosites supporting a connection of AKT to excitation–contraction coupling. Phosphoproteins identified included L-type calcium channel, ryanodine receptor, junctophilin, histidine-rich calcium binding protein, phospholamban, heat shock protein beta-6, and Ca2+/calmodulin-dependent kinase II. In conclusion, AKT isoform-specific knock down combined with quantitative phosphoproteomics provided a powerful strategy to unravel AKT isoform-specific signaling.
phosphoproteome of HL-1 cardiomyocytes carrying AKT1 or AKT2 isoform-specific knock down, respectively. We combined stable isotope labeling with high resolution mass spectrometry and identified 377 regulated phosphopeptides. Although AKT1 is expressed at 4-fold higher levels, insulin stimulation mainly activated AKT2, which might in part rely on a preferred interaction of AKT2 with the mammalian target of rapamycin complex 2. In line with this result, the highest number of regulated phosphopeptides was identified in the AKT2 knock down cells. Isoform-specific regulation of AKT targets not previously described could be observed, and specific regulation of indirect target sites allows a deeper insight into affected biological processes. In the myocardial context, we identified many phosphosites supporting a connection of AKT to excitation–contraction coupling. Phosphoproteins identified included L-type calcium channel, ryanodine receptor, junctophilin, histidine-rich calcium binding protein, phospholamban, heat shock protein beta-6, and Ca2+/calmodulin-dependent kinase II. In conclusion, AKT isoform-specific knock down combined with quantitative phosphoproteomics provided a powerful strategy to unravel AKT isoform-specific signaling.




掃碼咨詢微信客服

掃碼咨詢微信客服
©Copyright 上海雅吉生物科技有限公司 All Rights Reserved. ICP備案號:滬ICP備18032507號-1 技術(shù)支持:阿儀網(wǎng)
